Which of the Atoms Listed Below Has the Smallest Radius
What has the largest atomic radius F F Cl or Cl. The general electron configuration for atoms of all elements in Group5A is.

The Parts Of The Periodic Table
Which of the elements listed below has the smallest first ionization energy.
. This problem has been solved. Helium has the smallest atomic radius. Which of these choices is the general electron configuration for the outermost electrons of elements in the alkaline earth group.
Solve any question of Classification Of Elements And. A B Ge C D C P O 35. Consequently the ion with the greatest nuclear charge Al 3 is the smallest and the ion with the smallest nuclear charge N 3 is the largest.
A C b O c P d Ge e Se By signing up youll get. Which Verb makes an incorrect shift in tense. Which of these atoms has the smallest radius.
Which of these atoms has the largest radius. Sulfur O Chlorine Oxygen Fluorine. I Na Ba Cl Mg.
Acylinder is filled with 200 moles of nitrogen 300 moles of argon and 500 moles of helium. Which of the elements listed below has the smallest atomic radius. The monopolistic competition market structure is characterized by.
Which of the atoms listed below would have the smallest atomic radius. See the answer See the answer See the answer done loading. The branches creaked gently in the breeze.
Arrange the following ions in order of decreasing ionic radius. I Na Ba Cl Mg. Hence among given options B3 is smallest.
If the gas mixture is at stp what is the partial pressure of the argon. Few firms and similar products. Three of these points are the vertices of a.
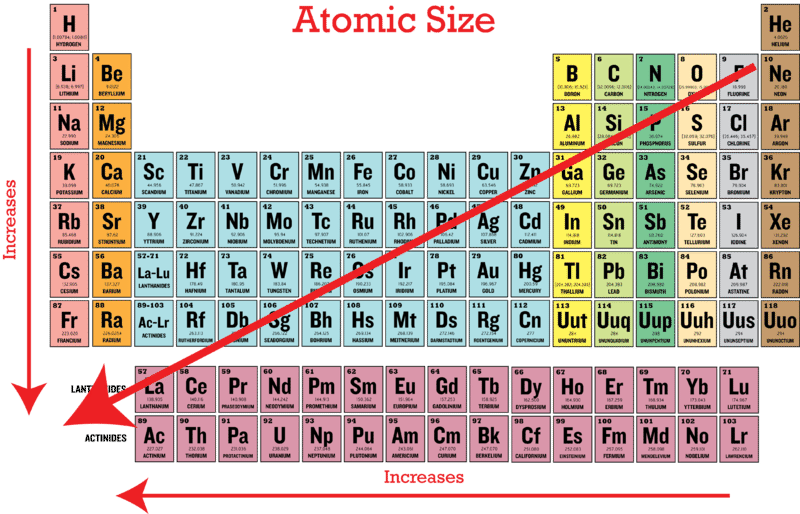
Which of the following element has the smallest atomic radius. Therefore fluorine has the smallest atomic radius. Atomic radii become larger as you go from top to bottom of the periodic chart but they get smaller as you go from left to right.
Which of the elements listed below has the greatest atomic radius. At the same time protons are being added to the nucleus. This is due to the extra screening by the 3d electrons which offset the increasing pull by the additional protons going from Fe to Co and Ni on the outer 4s electrons.
The neon atom in this isoelectronic series is not listed in Table 283 because neon forms no covalent or ionic compounds and hence its radius is difficult to measure. Therefore the elements with the. The bond length between atoms A and B is the sum of the atomic radii d AB r A r B.
C N O S Answer. Correct option is C Fe Co and Ni are transition metals in the same period where the atomic radii do not vary much. Nitrogen atomic radius.
Far off in the. I1 7863 kJmol I2 1580 kJmol I3 3230 kJmol I4 4360kJmol I5 16000 and I6 20000 kJmol. Which of the atoms listed below has the smallest radius.
CrystalMaker uses Atomic-Ionic radii data from. Which of the following atoms listed below will have the smallest radiusNaTeAlPAs Can you please explain how we should look at the periodic table to determine the answer. Which of the atoms listed below has the smallest radius.
Chemistry questions and answers. 3 Show answers Another question on Chemistry. K has the largest radius.
Which one of these ions has the largest radius. Select True or False. Which ion has the largest radius.
Few firms and a homogeneous. Consider the element with the electron configuration Xe6s2 4f7. Which of the atoms listed below would have the smallest atomic radius.
Among Li B3 B3 is smaller as it has higher nuclear charge due to which its size is reduce as electrons are strongly attracted. Al 3 Mg 2 Na O 2. A Al B C As D P Te B.
Which of the following atoms has the smallest radius. Six points are equally spaced around a circle of radius 1. An atomic radius is the radii of an isolated non.
Which of the elements listed below has the highest first ionization energy. I do not understand where to begin in analyzing radius size. I guess the options are F F- Cl and Cl-.
The following successive ionization energies correspond to an element in the third row of the periodic table. 97 rows For example the atomic-ionic radius of chlorine Cl - is larger than its atomic radius. Out of these Cl- has the largest ionic radius and Cl has the largest atomic radius.
The radii of ions are always. Based on this pattern of ionization energies identify the element. 60 Which of the atoms listed below has the smallest radius A Al B P C As D Te E from BIOCHEM 1005 at University of New England.
A K B Ca C Br D Kr E Zn Medium Solution Verified by Toppr Correct option is D As you go across a period electrons are added to the same energy level. Atomic radius in nanometers.
Atomic Radius Trend Periodic Table Chemtalk
No comments for "Which of the Atoms Listed Below Has the Smallest Radius"
Post a Comment